Background
In cold agglutinin mediated autoimmune hemolytic anemia (cAIHA), anti-red blood cell autoantibodies lead to complement-mediated hemolysis with or without symptoms of acrocyanosis after exposure at low temperatures. cAIHA can be divided into cold agglutinin disease (primary CAD) and cold agglutinin syndrome (CAS). The latter is secondary to diseases such as B-cell malignancies including CLL, infections or autoimmune disorders. In primary CAD, more than 90% of patients have a monoclonal IgM (mostly low level) and often a small bone marrow B-cell clone. There is no approved treatment. For patients with significant hemolytic anemia or acrocyanosis despite thermal protection, rituximab is the most accepted first line treatment with an overall response rate of 50% and median duration of response <1 year. Cytotoxic combinations such as rituximab-bendamustine produce more sustained remissions, although with concerns for long-term adverse effects and stem cell toxicity. Studies involving complement inhibitors are showing promising results on hemolysis, although cold induced peripheral symptoms (IgM mediated rather than complement-mediated) will not improve. Recent international guidelines on cAIHA suggest treatment with the Bruton tyrosine kinase (BTK)-inhibitor ibrutinib in refractory patients with cAIHA (Jäger et al Blood Rev 2020). Indeed, the underlying pathophysiology of cAIHA suggest that BTK inhibition could be effective.
Aims
To evaluate the efficacy of ibrutinib on anemia, hemolysis and acrocyanosis in patients with cold agglutinin-mediated AIHA (CAD/CAS).
Methods
An international retrospective study was undertaken of cAIHA patients (CAD/CAS) treated with BTK inhibition using a preformed questionnaire. For eligible patients, laboratory and clinical data regarding underlying disease, bone marrow pathology, hemolytic parameters and patient-reported acrocyanosis were collected at diagnosis, 30 days, 3 months, 6 months and 12 months and last date of follow up. Hemoglobin (Hb) response was considered none (NR), partial (PR, >2 g/dL Hb increase or >10g/dL) or complete (CR, >12g/dL). Adverse events were graded according to the Common Terminology Criteria, version-5.0 (2017).
Results
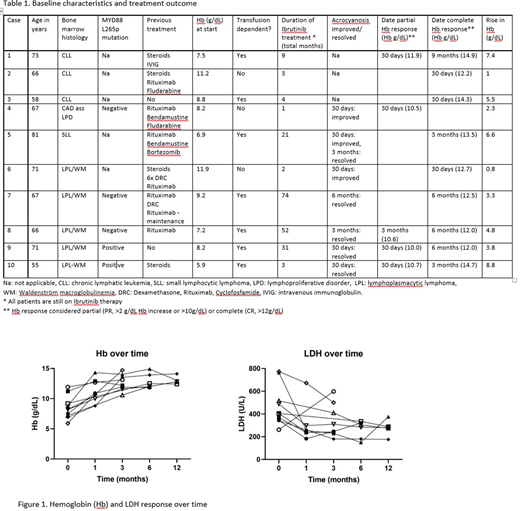
So far, 10 patients with cAIHA treated with a BTK-inhibitor (all involving ibrutinib) could be included in the study. Patients were followed from April 2014 until June 2020 at 5 centers (Italy (2), Norway, The United Kingdom and The United States). Median duration of follow up was 20 months (1-74 months). The main findings are summarized in table 1. The indication to start treatment was cAIHA based in all but 1 case (CLL). Median previous number of therapies was 2. All patients had a complement-mediated hemolytic anemia, 7 were transfusion-dependent, and 7 reported symptoms of acrocyanosis at the initiation of ibrutinib.
After initiation of ibrutinib, all patients showed an improvement in hemoglobin (Median rise: 4.4 g/dL) resulting in 1 PR and 9 CR. All 7 transfusion-dependent patients became transfusion independent (5 within 30 days). In all but 1 patient, markers of hemolysis (LDH, bilirubin) improved after initiation of ibrutinib (see Figure 1). All 7 patients with acrocyanosis reported clear clinical improvement, with complete resolution of symptoms in 5. There was 1 adverse event (grade 1 bleeding). Data collection is still ongoing and future updates are expected.
Conclusion
Data show that ibrutinib is effective in the treatment of cAIHA with a notable and brisk improvement of both the hemolytic anemia as well as the cold induced peripheral symptoms. Although preliminary, these promising data support further research of BTK-inhibitor based treatment of cAIHA (CAD/CAS) in a prospective study.
Berentsen:Alexion, Apellis, Bioverativ and Janssen-Cilag: Other: Travel grants ; Alexion, Apellis, Bioverativ, Janssen-Cilag, True North Therapeutics: Honoraria; Apellis, Bioverativ, Momenta Pharmaceuticals and True North Therapeutics: Consultancy; Mundipharma: Research Funding. Castillo:TG Therapeutics: Research Funding; Pharmacyclics: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Kymera: Consultancy; Abbvie: Research Funding; Janssen: Consultancy, Research Funding. Treon:Bristol-Meyer-Squibb: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding. D'Sa:Sanofi: Honoraria; BeiGene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding.
BTK-inhibitors (ibrutinib/acalabrutinib) are not yet indicated for the use in (primairy) cold autoimmune hemolytic anemia (cAIHA). However it is indicated for use in Waldenstrom macroglobulinemia (WM) and chronic lymphatic leukemia (CLL). Here we report retrospective data on a cohort of cases treated with ibrutinib for cAIHA mostly secondary to WM or CLL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal